Digging deeper into the genetics of drought tolerance in wheat
The ability of wheat plants to survive and thrive relies most importantly on one key environmental factor — water. Even so, the average wheat plant grown in the Pacific Northwest (PNW) will likely experience drought at some point in its lifecycle. The ability of roots to sense and respond to water deficit is a key mechanism to ensure survival and, ultimately, secure stable yields. We have been investigating the genetic variation in wheat root systems for over a decade now, because we hope that understanding this hidden half of the plant will help unlock secrets to yield optimization in water-limited conditions.
Plant breeders take advantage of genetic variation from wild relatives and landraces to search for new ways to improve elite wheat varieties. Most of our research over the past funding cycle has focused on understanding responses to drought in Louise and an Iranian landrace called AUS28451. Louise is a PNW-adapted soft white wheat variety with good stripe rust resistance, whereas AUS28451 is a landrace that shows resistance to root lesion nematodes as well as increased lignin in the roots. Lignin is a phenolic compound that decorates secondary cell walls of plants, which acts to fortify the stem tissue and subsequently prevent lodging as well as lines the vascular tissue to prevent water loss and improve water movement.
Previous research in our lab found that Louise and AUS28451 lines have very different root architectures. Specifically, Louise showed decreased root biomass and increased root diameter compared to AUS28451. The research literature has shown that increased root biomass, area, and length are associated with drought tolerance. Recent studies have also shown plants with increased lignification in stems, leaves, and roots showed improved drought tolerance. But it is unclear how root traits, drought, and lignin are correlated. Moreover, it is not simple because increased lignin content also inhibits growth and has other unwanted consequences, like straw with more lignin breaks down more slowly.
The purpose of our recent work is two-fold. The first goal is to investigate whether lignin composition plays a role in drought stress in wheat varieties important to the Inland Pacific Northwest. The second goal is to measure how plants respond to drought on a molecular level.
First, we performed biochemistry experiments to further investigate not 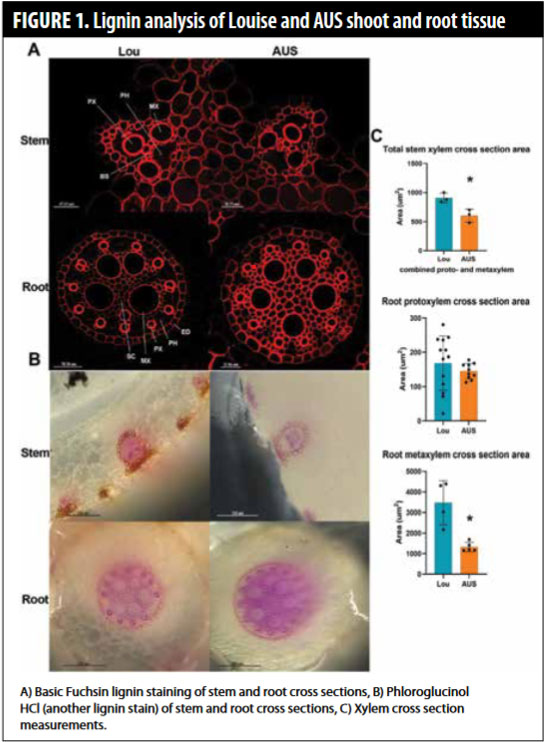 only total lignin, but also the chemical composition of lignin in Louise and AUS28451. This,we hoped, would shed light onto how lignin composition impacts drought response. We found Louise roots and shoots differed in the lignin cell wall composition and in modification of lignin in the cell wall compared to AUS28451 shoots and roots. We also looked at the spatial distribution of lignification in root cross-sections and found increased lignification in AUS28451 compared to Louise throughout the root vascular system (Figure 1). In addition, we found a decrease in the area of the xylem, the water-conducting cells of the root, in AUS28451 roots. Taken together, these data indicate and confirm that root traits, both for lignin content and vascular organization, highly differ between AUS28451 and Louise.
only total lignin, but also the chemical composition of lignin in Louise and AUS28451. This,we hoped, would shed light onto how lignin composition impacts drought response. We found Louise roots and shoots differed in the lignin cell wall composition and in modification of lignin in the cell wall compared to AUS28451 shoots and roots. We also looked at the spatial distribution of lignification in root cross-sections and found increased lignification in AUS28451 compared to Louise throughout the root vascular system (Figure 1). In addition, we found a decrease in the area of the xylem, the water-conducting cells of the root, in AUS28451 roots. Taken together, these data indicate and confirm that root traits, both for lignin content and vascular organization, highly differ between AUS28451 and Louise.
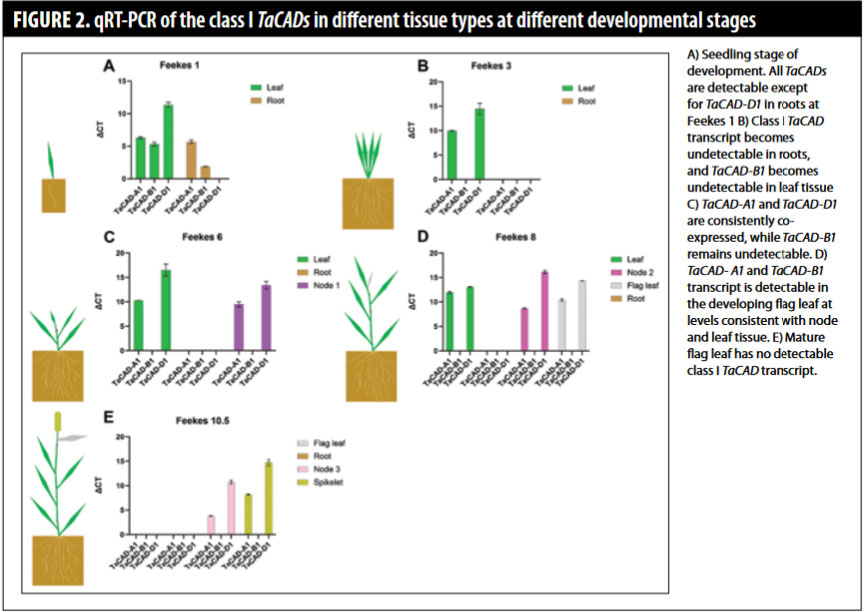 Next, we decided to investigate the genetics of lignification in wheat roots. To that end, we characterized the gene family that is known to encode the final step in the production of lignin monomer components — the CAD gene family. CAD stands for CINNAMYL ALCOHOL DEHYDROGENASE (CAD in italics indicates genes and nonitalicized references are proteins) and encodes an enzyme that makes the precursor molecules or monomers to the lignin polymer. We found that CAD genes are encoded by a large protein family with 47 members in wheat. We performed a genome-wide analysis and found their locations on the wheat chromosomes. We care about genes because they encode proteins. These proteins carry out the business of the cells and plant, like making cell walls, lignin, and responding to stressors in the environment. CAD proteins can be divided into three classes, or types of proteins. It is considered that only the Class I CADs are mostly responsible for lignin production in plants. Therefore, we performed expression analysis of the three Class I CAD genes in wheat called TaCAD-A1, TaCAD-1B, and TaCAD-1D. We detected expression of CAD genes throughout development, and found expression is mostly correlated with rapidly growing tissues. For example, all three TaCAD genes were expressed in shoots at Feekes 1 stage, and two of the three in roots at Feekes 1. However, later in development, we could not detect Class I CAD expression in the roots (Feekes 3 and later developmental stages). The TaCAD-A1 and TaCAD-1D genes are the most highly expressed genes throughout development (Figure 2).
Next, we decided to investigate the genetics of lignification in wheat roots. To that end, we characterized the gene family that is known to encode the final step in the production of lignin monomer components — the CAD gene family. CAD stands for CINNAMYL ALCOHOL DEHYDROGENASE (CAD in italics indicates genes and nonitalicized references are proteins) and encodes an enzyme that makes the precursor molecules or monomers to the lignin polymer. We found that CAD genes are encoded by a large protein family with 47 members in wheat. We performed a genome-wide analysis and found their locations on the wheat chromosomes. We care about genes because they encode proteins. These proteins carry out the business of the cells and plant, like making cell walls, lignin, and responding to stressors in the environment. CAD proteins can be divided into three classes, or types of proteins. It is considered that only the Class I CADs are mostly responsible for lignin production in plants. Therefore, we performed expression analysis of the three Class I CAD genes in wheat called TaCAD-A1, TaCAD-1B, and TaCAD-1D. We detected expression of CAD genes throughout development, and found expression is mostly correlated with rapidly growing tissues. For example, all three TaCAD genes were expressed in shoots at Feekes 1 stage, and two of the three in roots at Feekes 1. However, later in development, we could not detect Class I CAD expression in the roots (Feekes 3 and later developmental stages). The TaCAD-A1 and TaCAD-1D genes are the most highly expressed genes throughout development (Figure 2).
Following the characterization of the CAD gene family, we investigated which genes change in their expression in response to drought. We used a molecular technique called RNA sequencing that measures all the genes that change expression in response to drought. Specifically, we compared three wheat lines: Louise, AUS28451, and Chinese Spring (drought 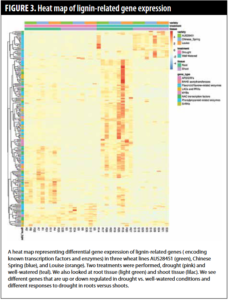 susceptible) and looked at how gene expression in each line changed when plants experienced drought stress. We compared both root and shoot responses to drought. We found thousands of genes that are differentially expressed in response to drought. To make meaning of these genes and to focus on our goal of looking at the lignin responses in response to drought, we focused on enzymes and transcription factor proteins that are known to be involved in lignin production or in regulating lignin production (Figure 3). These data show us that lignin genes are more highly expressed in growing tissues (well-watered conditions) — if you look at the histogram, you see more red color in the root and shoot tissues. Moreover, the genes that are responding in roots and shoots are different. In addition, there are variety-specific responses. Many of the same genes increase expression in all varieties but some are unique.
susceptible) and looked at how gene expression in each line changed when plants experienced drought stress. We compared both root and shoot responses to drought. We found thousands of genes that are differentially expressed in response to drought. To make meaning of these genes and to focus on our goal of looking at the lignin responses in response to drought, we focused on enzymes and transcription factor proteins that are known to be involved in lignin production or in regulating lignin production (Figure 3). These data show us that lignin genes are more highly expressed in growing tissues (well-watered conditions) — if you look at the histogram, you see more red color in the root and shoot tissues. Moreover, the genes that are responding in roots and shoots are different. In addition, there are variety-specific responses. Many of the same genes increase expression in all varieties but some are unique.
In summary, we have used biochemistry and genetics to try to understand how wheat plants respond to drought, how different varieties respond to drought, and how different tissues (roots vs. shoot) respond to drought. Overall, we found lignin is an important cell wall constituent and varies in wheat varieties, but lignin production is not necessarily regulated on the transcriptional level in response to drought. These experiments have yielded a wealth of data that will keep Washington State University researchers and students busy for years to come. By understanding which genes and proteins are important for stress responses, breeders can determine not only markers, but also genes and the proteins they make that enhance performance of wheat varieties. Finally, we are extremely grateful to you, the grain growers and commissioners who support our work on wheat, lignin, and drought.
This article originally appeared in the July 2024 issue of Wheat Life Magazine.

Luigi Peracchi
Graduate Student, Washington State University Sanguinet Lab

Karen Sanguinet, Ph.D.
Karen Sanguinet is an associate professor at the Washington State University College of Agricultural, Human, and Natural Resource Sciences. Her research focuses on factors that modulate growth and development, particularly in response to the environment. She uses physiological, developmental, genetic and genomic approaches to study plant root development and architecture. Read more about Dr. Sanguinet.
