Optimizing wheat production in acid soils
By Joao Antonangelo
Wheat stands tall as one of the staple 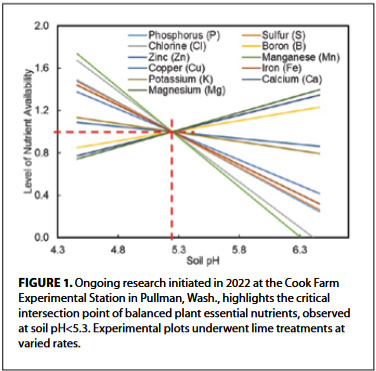 grains nourishing billions worldwide. Behind every loaf of bread and every bowl of pasta lies the dedication of farmers battling diverse challenges to ensure bountiful harvests. Among these challenges, acidic soils pose a significant hurdle, thwarting the full potential of wheat cultivation. However, with the right approach, even acid soils can yield flourishing wheat crops. Enter lime requirement assessment, a crucial step in soil management, where the base saturation (BS%) approach emerges as a beacon of efficiency, offering promising solutions to farmers worldwide.
grains nourishing billions worldwide. Behind every loaf of bread and every bowl of pasta lies the dedication of farmers battling diverse challenges to ensure bountiful harvests. Among these challenges, acidic soils pose a significant hurdle, thwarting the full potential of wheat cultivation. However, with the right approach, even acid soils can yield flourishing wheat crops. Enter lime requirement assessment, a crucial step in soil management, where the base saturation (BS%) approach emerges as a beacon of efficiency, offering promising solutions to farmers worldwide.
The acid soil dilemma
Acidic soils, characterized by low pH levels, hamper wheat production by impeding nutrient availability, hindering root growth, and promoting toxic aluminum (Al) accumulation. As a result, wheat yields dwindle, robbing farmers of their livelihoods and communities of sustenance. The conventional solution to combat acidity involves raising soil pH through lime application, a practice crucial for unlocking the soil’s fertility potential.
The pH target approach: a common pitfall
Traditionally, farmers have relied on the pH target approach to determine lime requirements, aiming to elevate soil pH to a predetermined level, often 6.5 to 7, a globally established range for optimal availability of essential nutrients. However, while this method has its merits, it often falls short in accurately addressing soil chemistry dynamics. Soil pH alone provides an incomplete picture of soil fertility, overlooking critical factors like cation exchange capacity (CEC) and BS% levels.
The base saturation approach
In contrast, the BS% approach offers a holistic perspective, considering not only soil pH but also the relative proportions of exchangeable cations — calcium (Ca), magnesium (Mg), and potassium (K) — to detrimental (or acidic) cations — Al and hydrogen (H). By striving for balanced cation ratios, this approach ensures optimal soil fertility, fostering ideal conditions for wheat cultivation.The benefits of the base saturation approach include:
- Precision in lime application. Unlike the pH target approach, which may lead to over- or under-liming, the BS% approach tailors lime application to specific soil needs, optimizing resource utilization, and minimizing costs for farmers.
- Improved nutrient availability. By targeting balanced cation ratios, the BS% approach enhances nutrient availability, fostering robust root development, and maximizing wheat yield potential.
- Long-term soil health. Beyond immediate pH correction, the BS% approach promotes long-term soil health, mitigating acidity-induced Al toxicity, and preserving soil structure for sustained productivity.
- Environmental sustainability. By promoting efficient lime utilization, the BS% approach minimizes environmental impact, reducing the risk of lime leaching into water bodies and mitigating carbon emissions associated with lime production.
Why this is true?
Because raising soil pH to the 6.5 to 7.0 range can potentially cause nutrient imbalances:
- Availability of nutrients. While some nutrients become more available to plants as soil pH increases, others may become less available. For instance, phosphorus availability tends to decrease as pH increases significantly, which can limit plant uptake (see Figure 1).
- Nutrient interactions. Soil pH influences the chemical reactions that govern nutrient availability and uptake. Altering pH can affect the interactions between different nutrients in the soil, potentially leading to imbalances. For example, high pH can increase the availability of Ca and Mg while decreasing the availability of other essential nutrients like zinc and copper (Figure 1).
Because wheat yields do not respond to lime application targeting pH 6.5 to 7.0:
- Optimal pH range. High pH
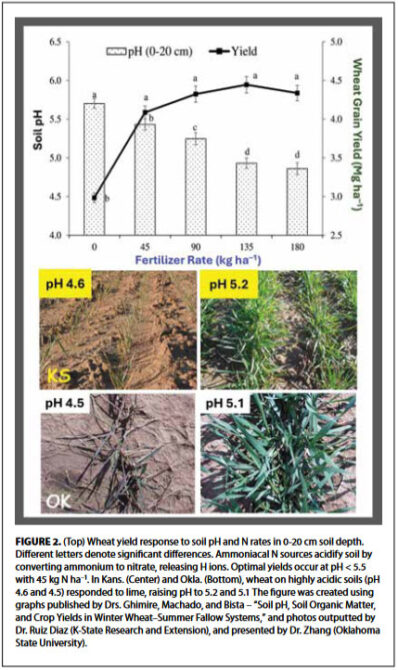 levels can lead to nutrient imbalances and reduced nutrient availability, thereby limiting yield potential. Therefore, applying lime to raise pH to the 6.5 to 7.0 range may not necessarily benefit wheat yields and could potentially hinder growth. Multiple studies conducted across the U.S. from the 1980s (Pacific Northwest) through the past decade (southern U.S.) have consistently demonstrated that regardless of whether the cultivar exhibits resistance or sensitivity to toxic aluminum and low soil pH, there is no observed increase in yield beyond a soil pH of 5.5 even with higher rates of lime application. Additional findings elsewhere in the U.S. (Columbia Basin, Oklahoma, and Kansas) are illustrated in Figure 2.
levels can lead to nutrient imbalances and reduced nutrient availability, thereby limiting yield potential. Therefore, applying lime to raise pH to the 6.5 to 7.0 range may not necessarily benefit wheat yields and could potentially hinder growth. Multiple studies conducted across the U.S. from the 1980s (Pacific Northwest) through the past decade (southern U.S.) have consistently demonstrated that regardless of whether the cultivar exhibits resistance or sensitivity to toxic aluminum and low soil pH, there is no observed increase in yield beyond a soil pH of 5.5 even with higher rates of lime application. Additional findings elsewhere in the U.S. (Columbia Basin, Oklahoma, and Kansas) are illustrated in Figure 2. - Other soil factors. Soil fertility and productivity are influenced by a combination of factors beyond just pH, including nutrient levels, soil structure, moisture availability, and microbial activity. If these factors are not optimal, wheat yields may not respond significantly to lime application targeting specific pH levels.
Keep in Mind
In global wheat farming, soil pH is still a critical factor impacting nutrient availability and crop performance. Despite similar pH levels, soil nutrient balances vary due to factors
like fertilization history and microclimates. Establishing a universal pH standard is challenging, but a BS% can offer a more standardized approach, though wheat cultivars must be considered. Understanding local conditions and cultivar needs is key to optimizing soil health and ensuring sustainable yields worldwide.
This article originally appeared in the July 2024 issue of Wheat Life Magazine.

Joao Antonangelo, Ph.D.
Joao Antonangelo is an assistant professor in soil chemistry and is the Washington Wheat Distinguished Professor at Washington State University. His research emphases are on soil organic matter dynamics and soil biochemistry in direct support of healthy and sustainable grain production. Read more about Dr. Antonangelo.
